





Dell and Apple recently announced recalls of 5 million Sony laptop batteries. The recall was prompted by safety issues caused by batteries melting, and in some cases, even catching on fire. While the incidents aren't widespread, the very real danger posed by laptop batteries unpredictably catching on fire has consumers worried.
Here's a look at the problem, how batteries work, and steps you can take to maximize your safety.
Why are batteries catching on fire?
There are two reasons that this large batch of Sony batteries are prone to meltdown. The first is a manufacturing problem exclusive to Sony, the second is the flammable nature of materials used in Lithium Ion (LiIon) batteries.
Fortunately, there is a lot of good news: the problem is well understood and isolated to specific batteries that were built improperly. Anyone with a recalled battery should simply get a replacement.
Dell Recall
Apple Recall
Correctly built LiIon batteries are very safe if handled properly. Additionally, the new generation of Lithium Polymer (LiPoly) batteries, used by Apple in the MacBook and MacBook Pro, don't use the same flammable materials as the older generation of LiIon batteries. That makes newer LiPo batteries even safer.
How Batteries Work
Batteries store electricity by converting electrical charge into chemical energy. The basic principle of all batteries involves separating two different metals in a solution, or electrolyte. The electrolyte causes a chemical reaction between the two metals, and the resulting exchange of charged ions can be captured and used to drive electric devices.
A battery can be as simple as a potato with two electrodes stuck into it; the potato acts as an electrolyte for the two metal electrodes, and can provide enough power to run a digital clock.
Two Potato Clock technology is available from Amazon for $15. The catch, of course, is that users have to keep replacing the potato. Since running a laptop would require lots and lots of potatoes, we need better power technology than vegetables can provide.
Lead Acid
Using a more efficient and longer lasting electrolyte, batteries can be designed for a variety of specific purposes. Car batteries use lead acid technology, which can provide a burst of electrical energy needed to start a car's engine, but must be frequently and continuously recharged or they lose their power capacity.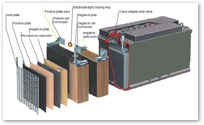

That's no problem for a car, which runs around generating plenty of excess electricity as its engine runs. Cars also have little problem carrying around a very heavy lead battery.
For other consumer applications, lead acid batteries are less than ideal. Apple's 1989 Macintosh Portable used a lead acid battery that contributed most of the machine nearly 16 pounds (over 7 kg). It did provide 10 hours of use however.
Nickel Cadmium
A newer battery technology using Nickel and Cadmium for the two metal electrodes resulted in much lighter devices. Nickel Cadmium (NiCd) batteries are popular in small devices such as RC cars, power tools, cordless phones, and other rechargeable battery applications.

However, NiCd battery technology has its own flaws. It tends to lose 20% of its charge per month, even when under no load. It supplies a lower voltage rate, and is prone to the "memory effect," where a battery fails to fully recharge after being partially discharged and recharged. All those factors made NiCad batteries poorly suited to laptops.
NiCad is also expensive to manufacture, and cadmium is toxic. Batteries should never be thrown in the trash, and particularly not NiCd batteries. NiCd batteries also produce hydrogen or oxygen gas if over charged, causing the possibility of rupture or explosion.
Nickel Metal Hydride
A new generation of batteries improved upon NiCd by using a hydride absorbing alloy in place of the toxic heavy metal cadmium. Nickel Metal Hydride (NiMH) batteries provide two to three times more capacity than NiCd and suffer less from the memory effect. That resulted in NiMH being the first good battery technology for early laptops, and was used by Apple's original line of PowerBooks.
NiMH still had the same gassing problem when overcharged, so NiMH battery chargers require smart controllers to prevent this from happening. NiMH batteries also self-discharge over time.
Lithium Ion
The demand for longer lasting batteries that could maintain their charge better and didn't suffer from the memory effect lead Sony to developing consumer Lithium Ion (LiIon) batteries in the early 90's. LiIon batteries are much lighter than previous generations of batteries, and don't have the same gassing, memory, or self-discharge problems.
Of course, Lithium Ion batteries have their own set of problems. First, LiIon batteries age over time, regardless of whether they are used or not. With that in mind, LiIon batteries should only be bought when needed, and not stocked in inventory or for long term storage.
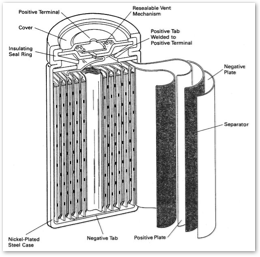 Unlike most other consumer batteries, LiIon batteries will also suffer permanent damage if charged down completely. That's why laptop computers shut down before the battery is completely run down, and why LiIon batteries are not sold directly to consumers as cell batteries; all LiIon batteries are packaged in a battery pack with smart controller to prevent users from running them completely dead. This smart controller uses a small bit of current to manage the battery level, so a LiIon battery shouldn't be left on the shelf uncharged, or it will eventually kill itself while performing its suicide watch.
Unlike most other consumer batteries, LiIon batteries will also suffer permanent damage if charged down completely. That's why laptop computers shut down before the battery is completely run down, and why LiIon batteries are not sold directly to consumers as cell batteries; all LiIon batteries are packaged in a battery pack with smart controller to prevent users from running them completely dead. This smart controller uses a small bit of current to manage the battery level, so a LiIon battery shouldn't be left on the shelf uncharged, or it will eventually kill itself while performing its suicide watch.Taking apart a LiIon laptop battery reveals a number of cell batteries installed inside. The individual cells of a LiIon battery are packaged in a cylinder to separate its layers: two metal electrodes with a lithium salt suspended in an organic solvent acting as the battery's electrolyte. These layers are tightly wrapped into the conventional cylinder battery cell shape and encased in a metal shell.
Lithium is highly reactive, so LiIon batteries use fuses and electronics to prevent overcharging and overheating. However, battery users have to supply some intelligence as well. Batteries can not be exposed to physical damage or excessive heat, such as being left in a hot car or in direct sunlight. They obviously can't be thrown in the trash or incinerated. Batteries can be taken to a number of recyclers, including Radio Shack, who accepts dead batteries for free.
Lithium Polymer
The next generation of lithium batteries embeds the lithium salt in a solid polymer. This improves upon LiIon technology by limiting the potential for exposure of the flammable lithium salt. Additionally, Lithium Polymer (LiPoly) batteries do not require layers of electrodes to be tightly wound into a cylinder cell shape.
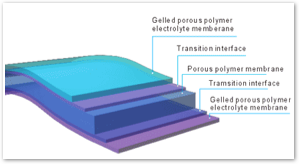 Instead, LiPoly batteries can be custom fit into any shape desired, allowing for much thinner designs. Because there is less wasted space surrounding the cases of individual cells, nor any metal casings, LiPoly offers much higher energy density as well.
Instead, LiPoly batteries can be custom fit into any shape desired, allowing for much thinner designs. Because there is less wasted space surrounding the cases of individual cells, nor any metal casings, LiPoly offers much higher energy density as well.Don't Panic!
So there you have it: posting photos of flaming laptops on the Internet should soon be a thing of the past.
Defective LiIon batteries should be returned in the recall, and all other batteries should be kept cool and charged to extend their lifespan and prevent untimely battery death.
As for the actual danger posed by exploding laptop batteries: since there have been fewer than 50 incidents of flaming batteries reported, there are clearly better things to worry about.

| | Comment Preview
 Read more about:
Read more about:

 Send |
Send |

 Subscribe |
Subscribe |
 Del.icio.us |
Del.icio.us |
 Digg |
Digg |
 Furl |
Furl |
 Reddit |
Reddit |
 Technorati
Technorati
Click one of the links above to display related articles on this page.
Exploding Battery Panic!
Saturday, August 26, 2006






Ad







